| Fatty
Acids |
|
| |
|
 |
|
Fatty
acids are carboxylic acids with hydrocarbon chains of 4 to 36 carbons.
In some fatty acids, this chain is fully saturated (contains no double
bonds) and unbranched; others contain one or more double bonds and are
unsaturated ones. |
|
The
physical properties of the fatty acids, and of compounds that contain
them, are determined by the length and degree of unsaturation of the
hydrocarbon chain. The nonpolar hydrocarbon chain accounts for the poor
solubility of fatty acids in water. The longer the fatty acyl chain
and the fewer the double bonds, the lower the solubility in water. The
carboxylic acid group is polar (and ionized at neutral pH), and accounts
for the slight solubility of short chain in water. |
|
In the fully saturated compounds, free rotation around
each of the carbon-carbon bonds gives the hydrocarbon chain great flexibility;
the most stable conformation is this fully extended form, In which the
steric hindrance of neighboring atoms is maximized. In a unsaturated
fatty acids, a cis double bond forces a kink in the hydrocarbon
chain, and fatty acids with one or more kinks cannot pack together as
tightly as fully saturated fatty acids and their interactions with each
other are therefore weaker. |
|
The melting points of fatty acids and of compounds that contain them
are also strongly influenced by the length and degree of unsaturation
of the hydrocarbon chain. At room temperature (25°C), the saturated
fatty acids have a waxy consistence, whereas unsaturated fatty acids
are oil liquids because it takes less thermal energy to disorder its
poorly ordered arrays of unsaturated fatty acids. |
|
In
the figures below, take a look in the carboxyl groups represented in
blue. |
|
Fatty
Acids |
|
|
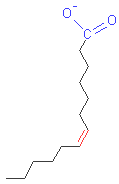 |
Saturated
Fatty Acid |
Unsaturated
Fatty Acid |
