

Lipids catabolism
Oxidation of polyunsaturated Fatty Acids
 |
|
| |
|
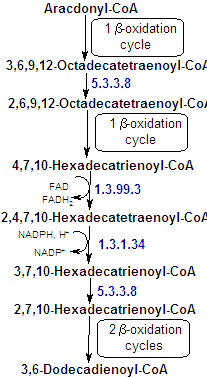
| Almost all unsaturated fatty acids of biological origin contain only cis double bonds. Beacuse of this, an aditional enzyme is required: enoyl-CoA isomerase, that converts the Δ3-cis-acyl-CoA formed during these fatty acids degradation to a Δ2-trans-acyl-CoA. Such compounds are normal substrates of enoyl-CoA hydratase so that b oxidation can the continue. Since this there's no need of forming a double bond by dehydrogenation, there's no FADH2 prodution by acyl-CoA dehydrogenase, and so the energy produced is smaller. |
|
Additional double bonds occur at three carbon intervals and are therefore never conjugated. Double bonds at these positions in fatty acids pose two problems for the b-oxidation pathway that are solved through the actions of three additional enzymes. The first problem is the formation of a Δ3-cis-acyl-CoA, and as said is solved by the action of enoyl-CoA isomerase. |
|
|
The next problem is that the presence of a double bond at an even-numbered carbon atom results in the formation of 2,4-dienoyl-CoA, wich is a poor substrate for enoyl-CoA hydratase. However, NADPH-dependent 2,4-dienoyl-CoA reductase reduces the Δ4 double bond. The mammalian reductase yields trans-3-enoyl-CoA, wich, to proceed along the b-oxidation pathway, must first be isomerized by trans-2-enoyl-CoA isomerase. |
|