

Carbohydrate Metabolism
Pentose Phosphate Pathway
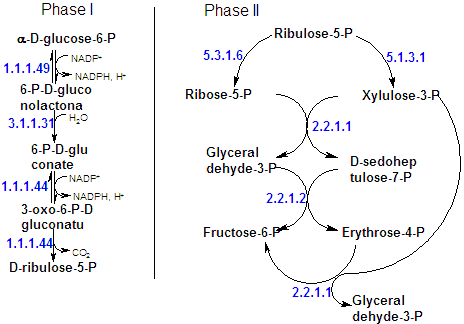 |
| |
| Besides ATP, cells need a different type of metabolic energy - reducing power. Many endergonic reactions require NADPH in addition to ATP. Despite their close chemical resemblance, NADPH and NADH are not metabolically interchangeable. Whereas NADH participates in utilizing the free energy of metabolite oxidation to synthesize ATP, NADPH is involved in utilizing the free energy of metabolite oxidation for otherwise endergonic reductive biosynthesis. |
| NADPH is generated by the oxidation of glucose-6-P via an altenartive pathway to glycolysis, the pentose phosphate pathway. |
| The overall reaction is: |
| 3Glucose-6-P + 6 NADP+
+ 3H2O |
| However, the pathway may be considered to have three stages: |
1-Oxidative reactions - glucose-6-P oxidation to yield NADPH and ribulose-5-P. |
3G6P + 6 NADP+
+ 3H2O |
2-Isomerization and epimerization reactions - wich transform Ru5P either to ribose-5-P or to xylulose-5-P |
3Ru5P |
3-A series of C-C bond cleavage and formation reactions that converts two molecules of Xu5P and one molecule of R5P to two molecules of fructose-6-P and one of Glyceraldehyde-3-P |
R5P + 2Xu5P |
The net results of these reactions is the formation of two hexoses and one triose from three pentoses. The reactions of stages 2 and 3 are freely reversible so that the products of the pathway vary with needs of the cell, the carbon skeletons of sugars can be extensively rearranged to meet physiologic needs. |