

Sphingolipidis Synthesis
 |
| |
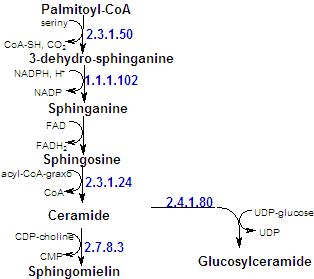
| The backbone of a sphingolipid is sphingosine, rather than glycerol. Palmitoyl-CoA and serine condense to form dehydrosphinganine (at endoplasmatic reticulum), wich is then converted to sphingosine. |
| In all sphingolipids, the amino group of sphingosine is acylated: a long chain acyl-CoA reacts with sphingosine to form ceramide (N-acyl-sphingosine). An alternative pathway was found in yeasts: |
| |
| Palmitoyl-CoA |
 |
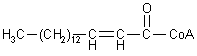 |
| trans-2-Palmitoleyl-CoA |
 |
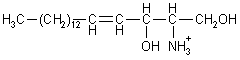 |
| Sphingosine |
 |
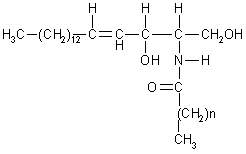 |
| Ceramide |
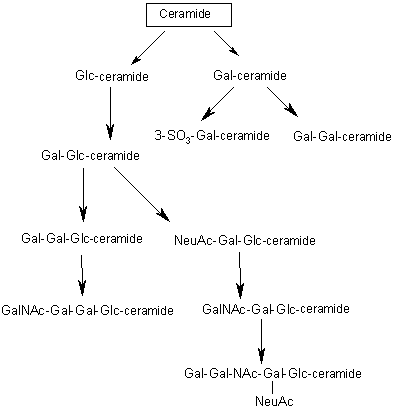
| The terminal hydroxyl group also is substituted. In sphingomyelin, the substituent is phosphorylcoline, wich comes from phosphatidylcoline. In a cerebroside, glucose or galactose is linked to the terminal hydroxyl group of ceramide. UDP-glucose or UDP-galactose is the sugar donor in the synthesis of cerebrosides. The enzymes that catalyze these reaction are glucotranferases, located in the lumen side of Golgi apparatus membrane. In a ganglioside, an oligosacharide is linked to ceramide by a glucose residue. |
 |
DHTML JavaScript Menu Courtesy of Milonic.com