

Amino acids Biosynthesis Glutamate, Arginine, Glutamine and Proline (from a-ketoglutarate) |
 |
| |
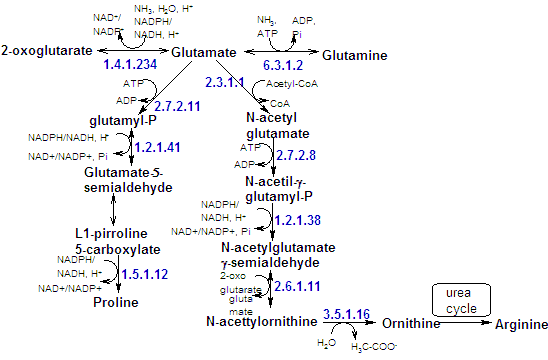
a-ketoglutarate is the a-keto acid that correspon to glutamate. Thereby, the synthesis of glutamate is a one-step transamination reaction. |
Glutamine is synthesized from glutamate amidation. Glutamine is the amino group donor in the formation of many biosynthetic products, as well as being a storage form of ammonia. |
Conversion of glutamate to proline involves the reduction of the g-carboxyl group to an aldehyde, followed by the formation of an internal Schiff base whose further reaction yields proline. |
Arginine is syntehsized from ornithine by the same reaction of the urea cycle. |