

| Phenylalanine, Tyrosine and Leucine degradation to Acetyl-CoA |
 |
| |
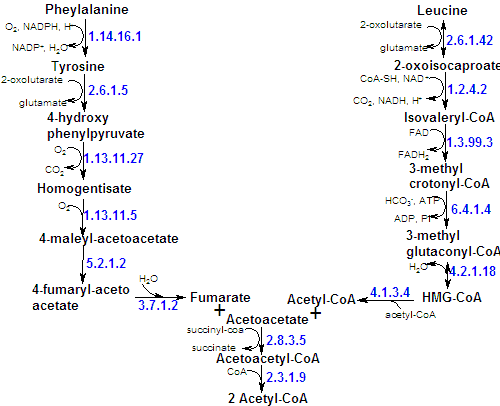
Since the first reaction in phenylalanine degradation is its hydroxylation to tyrosine, a single pathway is responsible for the breakdown of both these amino acids. The final product of the six-reaction degradation are fumarate (a citric acid cycle intermediate) and acetoacetate. They both are thus cetogenic and glicogenic. |
This series of reactions shows how molecular oxygen is used to break an aromatic ring. |
They are also precursors of the adrenal hormones epinephrine and norepinephrine, the neurotransmissor dopamine, and the black pigment of hair and skin - melanin. |
| The catabolism of the aromatic amino acids - tyrosine, tryptophan and phenylalanine – deserves atention not only because of their complexity (their side chains are responsable for the difficult on degradation) but also because they produce a great number of intermediates very important as other molecules precursors. |
| Phenylalanine has other less important degradation pathways in normal conditions, that can however be very important in pathologic conditions (lack of the major pathway). Phenylpyruvate is formed by transamination in these pathways. |
| See Leucine notes. |