| Oxidative
Phosphorylation |
|
| |
|

|
|
| |
|
|
|
|
| The oxidative phosphorylation or electron transport chain is the final step of all catabolism pathways. The NADH and FADH2 formed during the oxidation of various metabolic fuels are energy-rich molecules because each contains a pair of electrons having a high transfer potential. By oxidative phosphorylation, these electrons are donated to molecular oxygen by a series of electrons transporters. |
|
| The flow of electrons through protein complexes located in the inner membrane of mitochondria releases a large amount of energy, used to transport protons across the inner mitochondrial membrane from the matrix, a region of low [H+] to the intermembrane space, a region of high [H+] (active transport). A proton motive force is generated consisting of a pH gradient and a transmembrane electric potential. |
|
| ATP is synthesized when protons flow back to the mitochondrial matrix through a enzyme complex. So the proton motive force generated by the electron transport powers ATP synthesis. |
|
| |
|
| Electron transporters: |
|
The electron transfer can be: directly, as and hydrogen atom (one proton H+ plus one electron), or as an ion H- (one proton H+ plus to electrons). |
|
| Many proteins embedded in the inner mitochondrial membrane are organized into the four respiratory complexes of the electron-transport chain. NADH+ and FADH2 bring electrons from diverse catabolic pathways to this chain. The electron transporters include: membrane carriers (as quinones), cytochromes and iron-sulfur proteins. |
|
-Ubiquinone- it's capable of accepting and donating either one or two electrons because its semiquinone form is stable. This means its redution can occur in two different steps: |
|
-Receives the firs electron, being reduced to a semiquinone - UQH. |
|
-Receives the second electron yielding Ubiquinol - UQH2 |
|
The ubiquinone can so link a two-electron donor to an one-electron receptor. |
|
-Cytochromes - cytochromes are iron-proteins with different heme groups (cytochromes a, b or c). Cytochromes a and b are integral membrane proteins, and c is a peripheral membrane protein. |
|
| -Fe-S proteins - they are one-electron donors. |
|
| |
|
|
|
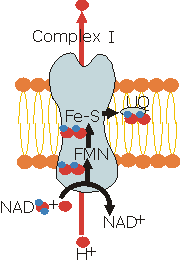
| General equation: NADH
+ H+ + UQ |
|
This complex contais
one molecule of flavin mononucleotide (FMN; a redox-active prosthetic
group that differs from FAD only by the absence of the AMP group), and
six to seven iron-sulfur clusters that participate in the electron transport
process. FMN semiquinone form, like ubiquinone, is stable. They both
provide an electron conduit between the two-electron donor NADH and
the one-electron acceptors, the cytochromes of the complex III. The
electron transfer is coupled to proton pumping (4 H+) by
a mecanism not known yet. |
|
| |
|
|
|
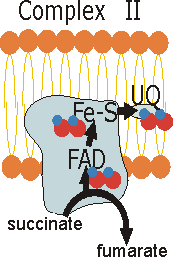
| Succinate dehydrogenase
(the enzyme that oxidates succinate) is the only enzyme of the Citrate
Cycle linked to the inner mitochondrial membrane - it's part of the
succinate-Q reductase complex (complex II), an integral protein
membrane. FADH2 does not leave the complex. Rather, its electrons
are transferred to Fe-S centers and then to UQ for entry into the electron
transport chain. This complex is not proton pump because the free-energy
change of the catalyzed reaction is too small. Consequently, less ATP
is formed from oxidation of FADH2 than from NADH. |
|
| |
|

|
|
The isoprenoid tail
makes ubiquinone nonpolar, wich enables it to diffuse rapidly in the
hidrocarbon core of the inner mitochondrial membrane. After receiving
the electrons from complex I or II, UQH2 moves to complex
III (cytochrome reductase) to give them to cytochromes (one-electron
acceptors). So, ubiquinol transfers one of its two high-potential electrons
to an Fe-S cluster in the reductase. This electron is then shuttled
sequentially to cit c1 and c, which carriers it away from this complex.
The other electron goes from the semiquinone to bL, bH, and back to
the UQ, that stay waiting in the wings, in the form of bound semiquinone.
A second molecule of UQH2 then reacts with the complex in
the same way as the first. However, this time bH reduces the bound UQH
(semiquinone) rather than UQ, to complete the Q cycle. Thus, two UQH2
are oxidized to form two UQ. The flow of a pair of electrons through
this complex leads to the effective net transport of 2H+
to the cytosolic side. |
|
| |
|

|
|
| Cytochrome c is a peripheral
membrane protein that is loosely bound to the outer surface of the inner
mitochondrial membrane. It can alternately binds to cytochrome c1 of
Complex III and to Complex IV (cytochrome c oxidase) and thereby
functions to shuttle electrons between them. The complex IV than catalyses
the one-electron oxidation of four consecutive reduced cytochrome c
molecules. These four electrons are funded into O2 to completely
reduce it to 2H2O and concomitantly pump protons from the
matrix to the cytosolic side of the inner mitochondrial membrane. |
|
| |
|
