|
ATP synthesis
|
|
|

|
|
|
|
|
|
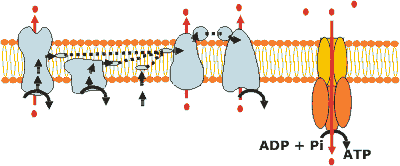
The electron transport chain does not produces ATP, but generate a proton motive force by pumping protons to the intermembrane space. |
| Through an enzyme complex, also located in the inner mitochondrial membrane - ATP synthase - these protons can flow back to the mitochondrial matrix, and the energy of this exergonic proccess is used to drive an endegornic one: the ATP synthesis from ADP and Pi. |
|
The proton-translocating ATP synthase contais two major structures (Fo and F1) and several different subunits. Fo is a transmembrane protein that contains a channel for proton translocation. F1 is a peripheral membrane protein. |
|
The mechanism for proton-driven ATP synthesis most accept was proposed by Boyer - the binding change mechanism. The three catalytic b subunits on F1 are not functionally equivalent at any particular moment. One catalytic site is in the O form, wich is open and has very low affinity for substrates. The second is in the L form, wich binds them loosely and is catalytically inactive. The third is in the T form, which binds them tightly and is active. When ATP is bounded to the T site, ADP and Pi then bind to the L site. Energy input by proton flux converts the T site into an O site, the L site into a T site, and the O site into an L site (T>O>L>T....). These conversions permit ATP to be formed from ADP and Pi at the new T site (former L site), and the release of the ATP from the T site (former O site). Protons flow from the Fo to the F1 side of the membrane only when O, L, and T interconvert. |
|
|