Pyrimidine Ribonucleotides Synthesis
|
Pyrimidine Ribonucleotides Synthesis |
|
|
 |
|
|
|
"de novo" synthesis |
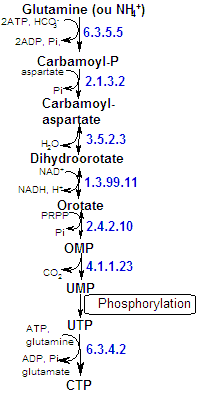
| The salvage of pyrimidines is less efficient because the bases, except uracil, can not be directly converted to nucleotides. Nucleosides must be sinthesized first. UMP (Uridine Mono-P) has a central role since it's the precursor of all the others pyrimidine nucleotides. |
|
The pyrimidines structure is more simple than the purines one, also it's their synthetic pathway. The pyrimidine ring is assembled first and then linked to ribose-P to form a pyrimidine nucleotide. The precursos of the pyrimidine ring are carbamoyl-P and aspartate. The first pyrimidine base formed, the one the ribose-P is linked to, is orotate (thus forming orotydilate - OMP). The other pyrimidine nucleotides are then synthesized from OMP. As IMP (purine), OMP concentrations within the cells is very small and it's not a nucleic acid component. |
|
|
Salvage pathways |
|
To convert pyrimidines to mononucleotides, nucleosides must be synthesized first. |
|
Many bacterias efficiently use pyrimidines bases to synthesize nucleotides. In constrast, these are loosely recycled by mammals. One of these salvage pathways is the synthesis of UMP from uracil, that either in bacterias and mammals is catalyzed by uridine phosphorylase and uridine kinase. The reactions are respectively: |
|
Uracil + ribose-1-P |
|
Uridine + ATP |
|
Mammals uridine phosphosrilase also accept deoxyuridine as substrate, and tymidine slowly, but not other nucleosides. |
|
Uridine kinase accepts only cytidine as substrate, but diverse nucleotides tri-P as phosphate donors. |