

Fatty acids synthesis
 |
| |
Fatty acid synthesis is not simply a reversal of the degradative pathway that takes place in the cytosol (in contrast with degradation, wich occurs in the mitochondrial matrix). The intermediates are covalently linked to the sulfhydryl groups of an acyl carrier protein (ACP), instead of to coenzyme A. |
The enzymes of fatty acid synthesis in higher organisms are joined in a single polypeptide chain called fatty acid synthase. The reductant is NADPH, whereas the oxidants in fatty acid degradation are NAD+ and FAD. |
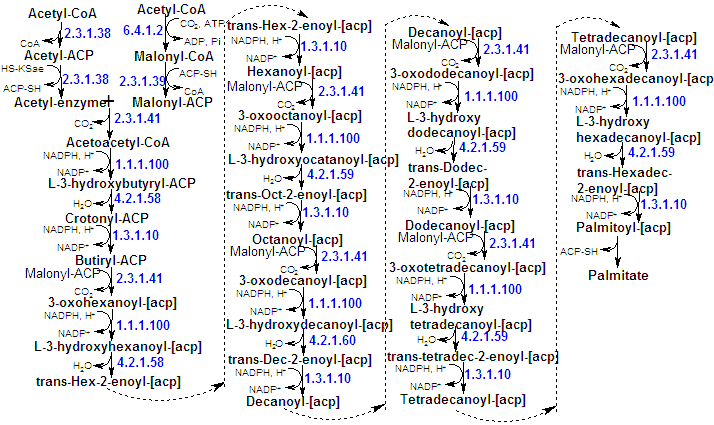
The growing fatty acid chain is elongated by the sequential addition of two-carbon units derived from acetyl-CoA, but the activated donor of two-carbon units in the elogation step is malonyl-ACP. The condensation reaction is coupled to the hydrolysis of ATP, thereby driving the reaction to completion. This proccess involves two steps: the ATP-dependent carboxylation of acetyl-CoA by acetyl-CoA carboxylase to form malonyl-CoA, and the exergonic decarboxylation of the malonyl group in the condensation reaction catalyzed by fatty acid synthase. Consequently, the CO2 taken up does not appear in the product fatty acid. Rather, the decarboxylation functions to drive the condensation reaction. |
The mechanism of synthesis occurs with a long flexible phosphopantetheine chain of ACP functioning to transport the substrate between the enzyme's various caralytic sites: |
-The "priming" of the condensating enzyme fllowed by formation of an acetyl-Cys residue (acetyl-CoA is the donor of a two-carbon unit only in this step, that occurs just once). |
| -The "loading" of the condensing enzyme by the formation of malonyl-CoA. |
| -The coupling of the acetyl group to the Cb of the malonyl group on the other subunit with the latter's accompanying decarboxylation so as to form acetoacetyl-ACP and free the active site Cys-SH group. |
| -The reduction, dehydration and further reduction followed by the transfer of the group formed to the Cys-SH of the first subunit. Thus the acetyl group with wich the system was initially primed has been elongated by a C2 unit. |
-The transfer of the group formed to the Cys-SH of the first subunit. |
The ACP group is "reloaded" with a malonyl group, and another cycle of C2 elongation occurs. This proccess occurs altogether seven times to form palmitoyl-ACP. Its thioester bond is then hydrolyzed by palmitoyl thioesterase yielding palmitate. This means that elongation by the fatty acid synthase complex stops upon formation of palmitate (C16). Further elongation and the insertion of double bonds are carried out by other enzyme systems. |
Stoichiometry of palmitate synthesis: |
| Malonyl-CoA
production: 7Acetyl-CoA + 7CO2 + 7ATP |
| 7
C2 cycles: 1Acetyl-CoA + 7 Malonyl-CoA + 14 NADPH + 14 H+
|
| Elongases and desaturases |
Palmitate (16:0) is the precursor of longer chain saturated and unsatturated fatty acids through the actions of elongases and desaturases. |
Elongases are present in both the mitochondrion and the endoplasmic reticulum. |
Unsatturated fatty acids are produced by terminal dessaturases. Mammalian systems contain four terminal dessaturases of broad chain-lenght specificities designated D9- D6-, D5- and D4-fatty acyl-CoA desaturases. |